Gleamer announces MDR-CE certification for two new solutions.

Gleamer, a leading healthcare technology company, is proud to announce the MDR CE Certification of two new solutions part of their BoneView solution: BoneView BoneMetrics and BoneView BoneAge. These solutions are designed to optimize readers’ workflow and improve accuracy.
The EU-MDR is a set of regulations established by the European Commission to improve the safety and quality of medical devices and ensure public health and patient safety through enhanced transparency for patients.
BoneView BoneAge automates Greulich & Pyle-based bone age assessment, which helps overcome the considerable reader variability of manual ratings. The solution is a breakthrough in healthcare technology and will significantly benefit healthcare providers and patients alike.
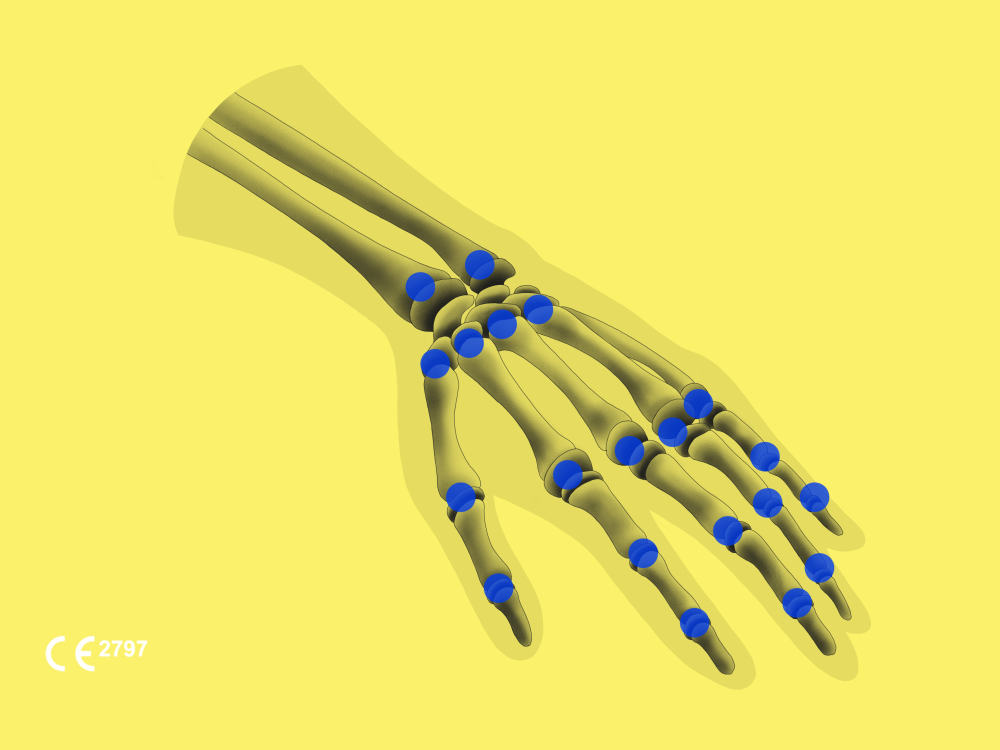
BoneView BoneMetrics provides automated MSK measurements for the pelvis, leg, feet, and spine, intending to save radiologists time and automatically generate a standardized report for orthopedic surgeons.
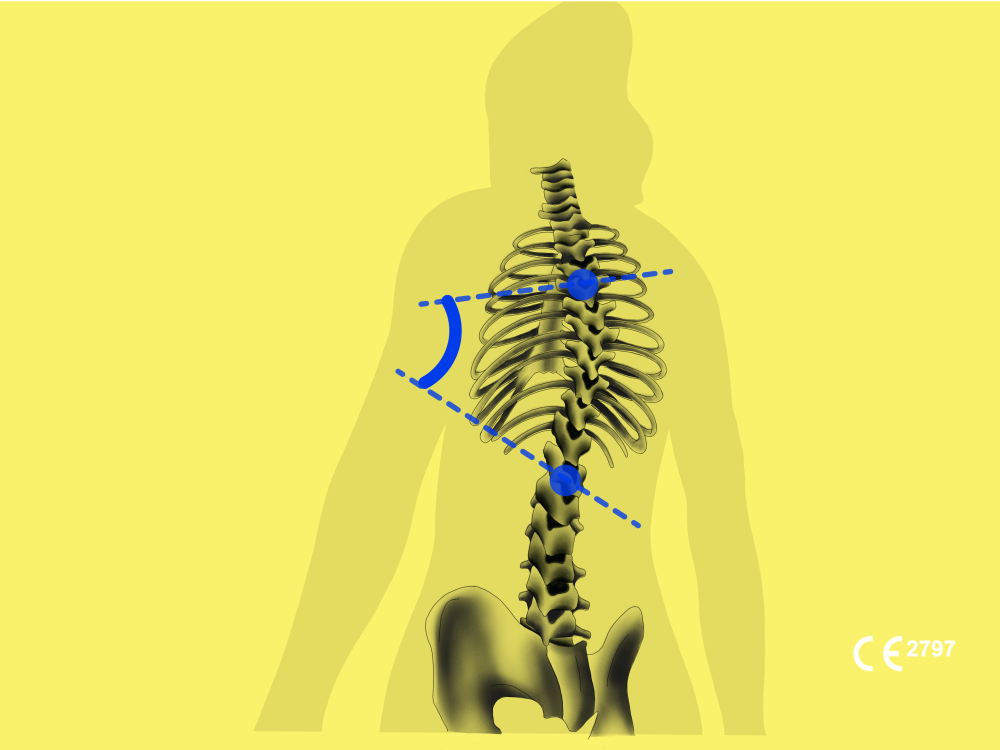
“We are thrilled to announce that our AI solutions have been certified as Class IIa medical devices under EU-MDR,” said Christian Allouche, CEO of Gleamer. “We are confident they will revolutionize the workflow of many centers.”
To learn more about these solutions and their clinical validation studies, request a demo from Gleamer. The studies’ abstract will also be presented at the ECR 2023.



